- 73 Posts
- 25 Comments

 1·1 year ago
1·1 year agoPretty sure you can download the maps ahead of time, GPS doesn’t require data, then upload the fixes when you get home.
Do it! it is easy to do at home! Just wear some gloves and safety glasses, those jars can easily shatter during the heating process if you use too hot of a heat source. I recommend a glass top electric stove, or put some kind of metal plate between your jar and the burner to help spread out the heat. Once you seal the jar, take it off the heat right away, so it doesn’t build pressure. I boiled mine for a few minutes before sealing to try and get some of the devolved gasses out, and lightly set the lid on top to help the steam push out all the air.

 182·1 year ago
182·1 year agoIt does. And Firefox is my default browser app.
Yes definitely. The pressure will drop along the vapor pressure curve all the way to the triple point, gently boiling all the way if you remove the heat from the vapor and not directly from the water.
Looks great! Nice work! What mount do you use?
Anything for posterity
Exactly. it was bottled at atmospheric pressure while it was boiling, so 1 atm and 100 degrees C. Check this graph to see the relationship between the water’s temperature and it’s pressure in the jar (since there is no air, only water vapor). If the vapor is condensed, then the pressure drops below the curve on the graph, that is, the pressure in the jar is lowered below the vapor pressure of the water. Any time the pressure is below the vapor pressure, the water will boil, releasing vapor, until the pressure is equal to the vapor pressure. The pressure does not become negative, it is still positive, just lower than the vapor pressure at the given temperature. You can get below the vapor pressure curve by changing the temperature too, which is what we usually do when boiling water at a pressure near 1 atm (760mmHg)
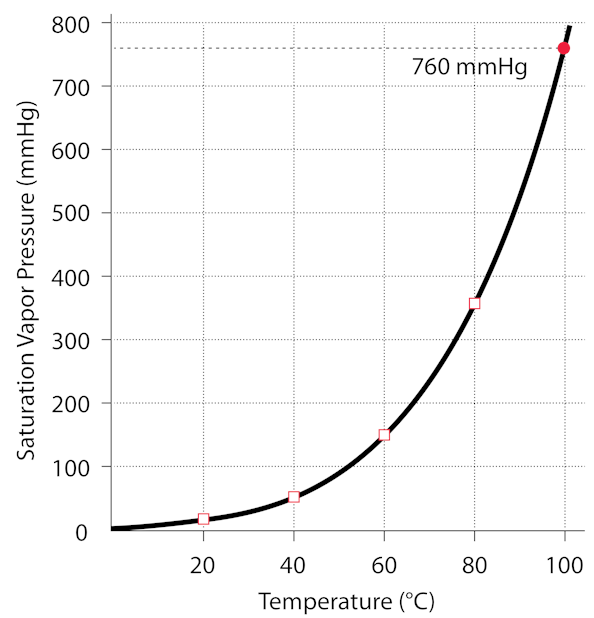
http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/watvap.html#c2
(1 atmosphere is ~760mmHg)
a slight aside, there is an important difference between the total pressure of the air, and the partial pressure of water vapor in the air. Inside the jar, the two are equal, but in a dry location (not humid) the partial pressure of water vapor is usually less than the vapor pressure of water at that temperature, but since the total large pressure of the atmosphere would not allow a pocket/bubble of very low pressure water vapor to form inside the bulk water, the water cannot boil, but it will evaporate at the surface anyway until the partial pressure of water is equal to the vapor pressure (very humid).
I decided to translate the worksheet into GLSL code on shadertoy. It was really cool to see the gradients and sub-coordinate systems represented by the intermediate variables in the calculation. Smoke and mirrors. Maybe you might have some insight into some of the calculations. https://www.shadertoy.com/view/cllBzM
Any ideas?
I was thinking of doing three separate GOL simulations, one on each RGB channel, and letting the colors mix that way into like 6 colors. right now, I clamp the pixel brightness values to 0 or 1, so that’s why it’s black/white, or rather black/green.

 1·1 year ago
1·1 year agoI thought there was something slightly peculiar about the narration.
Spinnaker


 21·1 year ago
21·1 year agoActually, this is more than just interesting. This is unsettling.

 101·1 year ago
101·1 year agoI hear you, but genetic change at the level of these diseases and traits can take on the order of hundreds of thousands of years or more to accumulate into meaningful trends. Social society is a part of that process, in the way it might be for other social animals. If social dynamics tend to result in communities harboring vulnerable individuals, then there is probably some selective advantage to that behavior, not the other way around.

 212·1 year ago
212·1 year agoThis is a common misconception. These traits are not likely due to modern medicine (which is very, very new compared to the scale of human evolution). The environment plays a big role, but there is always a distribution of traits in a normal population, some good, some bad. Not to mention that what we might be self-selecting for must change very rapidly as civilizations rise and fall, preferences shift like the winds, and ethics rapidly evolve. I think this misconception can be dangerous, because of what you mentioned. Eugenics.

 7·1 year ago
7·1 year agoIt’s very difficult and dangerous to be near an MRI ‘shutting down’. Assuming what you mean is turning of the magnet. The magnet is always on, its a coil of superconducting wire submerged in liquid helium with a very large permanent current flowing around it. In order to turn off the magnet quickly, the electric current must be quenched, which can happen if the coil every stops being a super conductor. The current starts heating the coil, causing the liquid helium to boil off, which doesn’t cool the coils as efficiently, and causes a rapid run-away effect where huge volumes of helium explode out of the machine, displacing all the breathable air in the room and blasting all the doors off their hinges, maybe even breaking windows. There’s a lot of energy stored in the coil. It’s not easy to turn it off.
Look up videos of MRI quenching
 1·1 year ago
1·1 year agoThis game is super fun

 1·1 year ago
1·1 year agoThe internet is a series of tubes.




















time for some kind of anonymizing location data sharing service, peer to peer or federated protocol? that might be interesting, or sketchy, not sure which.