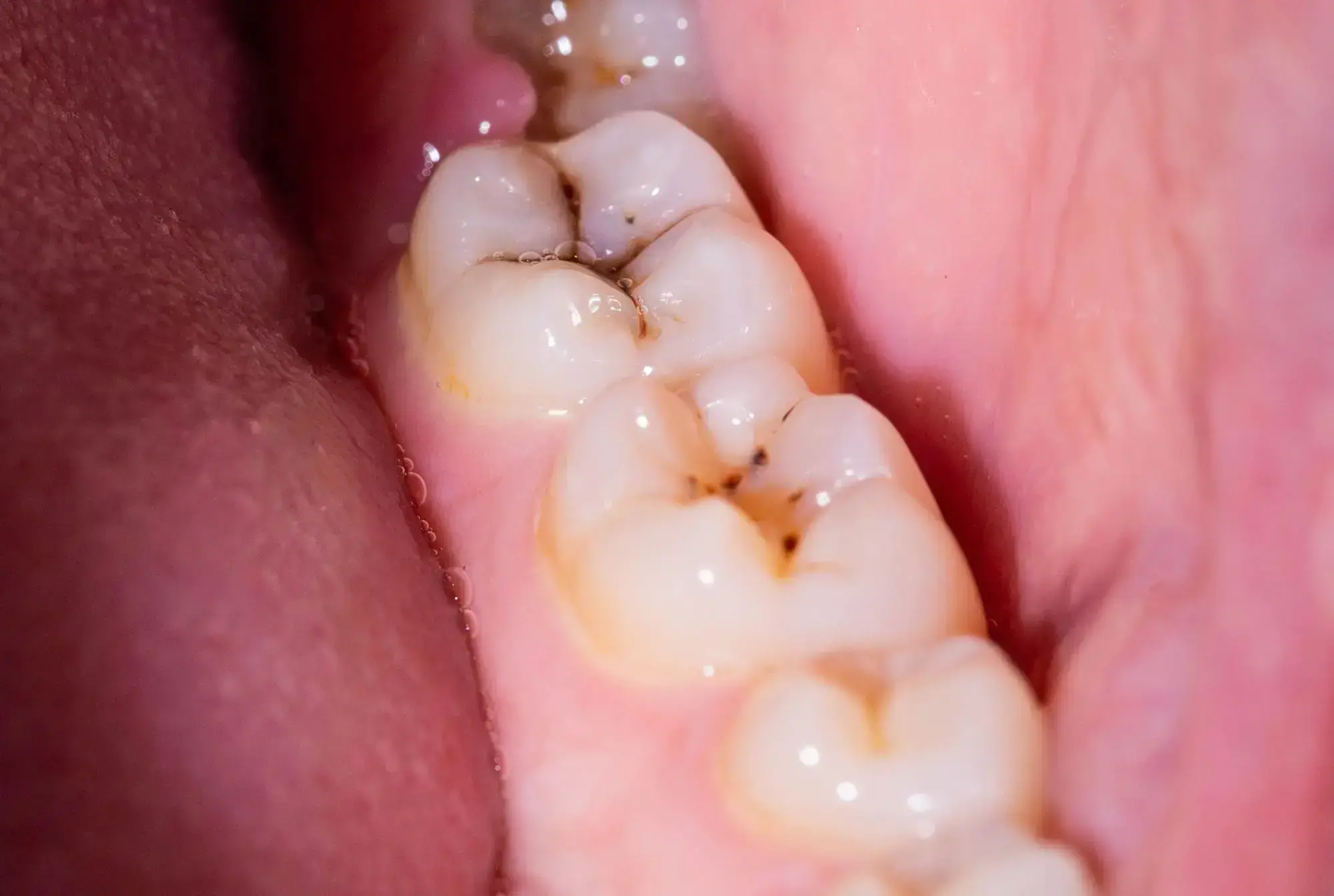
Scientists have discovered that the molecule DIM reduces biofilms causing dental plaque by 90%. Its addition to toothpaste and mouthwash could revolutionize dental hygiene. 3,3′-Diindolylmethane (DIM) decreased the Streptococcus mutans biofilm, a leading contributor to plaque and cavities, by 90%.
A significant portion of the global population experiences persistent issues with dental plaque and cavities or will face them at some time. While toothpaste, mouthwash, and routine dental visits help in prevention, there’s always room for improvement.
Researchers from Ben-Gurion University of the Negev, in collaboration with teams from Sichuan University and the National University of Singapore, have identified that 3,3′-Diindolylmethane (DIM) – a naturally occurring molecule also referred to as bisindole – can reduce biofilms responsible for plaque and cavities by a remarkable 90%.


I was hoping more for a to do list for my backyard, so I’ll have the molecule myself and not be beholden to Big Toothpaste.
I’m handy, I got all kinds of tech skills, but I’ve only dabbled in chemistry. I’m not uncapable, just green and rough around the edges. I’ve done a bunch of stuff in the past, like distillations, making sodium silicate (to seal up a forge), anodizing and electroplating, or bleach thru electrolysis. I’ve also used electricity to separate and collect gases. Hell I even took a fridge apart to use it’s old compressor to compress those gasses into different tanks - all in an effort to just not have to buy CO2, nitrogen or argon. Welding with hydrogen is triiiiicky, acetylene is much nicer. I even tried cold welding in a vacuum chamber, lol, but I need a better vacuum pump, probably a rotary one. I’ve made a bunch of different kinds of batteries and super capacitors.
I’ve thought about using calcium carbide to create my own acetylene, cuz I’ve got all the equipment I would need to capture and compress it, but y’know, I like living, and acetylene isn’t THAT expensive. Risk≠reward.
So I guess that’s my limit.
I do not recommend you to do this in your backyard. And if doing this, just as a curiosity, not as a reliable production method; this is NurdRage tier, not “make bleach at home and avoid Big Tech” tier. That said, on a theoretical level, the synthesis would be simply
You’ll probably get a 10% of junk compounds where the methyl attaches itself to the wrong carbon of the indole. Those could be separated by careful crystallisation.
As long as you take the proper security procedures, this is fairly safe. One of my uncles work with car soldering, and he produces acetylene at home.
Kind of hypocrite of me to say that, given that I have a scar on one of my arms from one of those reactions, but to be fair most people aren’t as stupid as a 14yo mixing calcium carbide and water inside a glass bottle, and closing it. (It was 20 years ago. My family still mentions this.)